
NCERT Solutions for Class 7 Science Chapter 5 Acids, Bases and Salts is an important chapter. This chapter focuses on Acids, Bases and Salts and many other important topics related to them such as crystallization. Here we are going to give a brief summary on these topics and all your doubts will be covered. We have also covered the questions and answers of this chapter.
Read more: NCERT Solutions For Class 7 Science Chapter 1 Nutrition In Plants
Class 7 NCERT Solutions for Class 7 Science Chapter 5 Acids, Bases and Salts: Introduction
You must have noticed that substances like lemon, tamarind, common salt, sugar and vinegar that we use in our daily life do not taste the same. Their different tastes are given below:-
| Substance | Taste (sour/bitter/ any other) |
| Lemon juice | sour |
| Orange juice | sour |
| Vinegar | sour |
| Curd | sour |
| Tamarind (imli) | sour |
| Sugar | sweet |
| Common salt | salty |
| Amla | sweet and sour |
| Baking soda | bitter |
| Grapes | sour |
| Unripe mango | sour |
| Cucumber | bitter |
We find that some of these substances taste sour, some taste bitter, some taste sweet and some taste salty.
NCERT Solutions for Class 7 Science Chapter 5 Acids, Bases and Salts
Acids
Substances that tastes sour for eg. curd, lemon juice, orange juice and vinegar contain acids. The acids give them a sour taste and the chemical nature of these substances is acidic. The word acid comes from the Latin word acere which means sour. Natural acids are present in these substances.
Bases
Substances that tastes bitter and feel soapy when we rub between our fingers for eg. baking soda are known as bases generally. The nature of such substances is said to be basic.
Indicators
Special types of substances are used to test whether a substance is acidic or basic. These substances are known as indicators. When we add an indicator to a solution containing acid or base, the indicators change their color.
Some naturally occurring indicators are –
- Turmeric
- Litmus
- China rose petals (Gudhal)
SOME COMMONLY OCCURING ACIDS AND BASES AND THEIR SOURCES.
| Name of acid | Found in |
| Acetic acid | Vinegar |
| Formic acid | Ant’s sting |
| Citric acid | Citrus fruits such as oranges, lemons, etc. |
| Lactic acid | Curd |
| Oxalic acid | Spinach |
| Ascorbic acid (Vitamin C) | Amla, Citrus fruits |
| Tartaric acid | Tamarind, grapes, unripe mangoes, etc. |
| Name of base | Found in |
| Calcium hydroxide | Lime water |
| Ammonium hydroxide | Window cleaner |
| Sodium hydroxide/ Potassium hydroxide | Soap |
| Magnesium hydroxide | Milk of magnesia |
NOTE: We should not taste unknown substances because they could harm us.
NCERT Solutions for Class 7 Science Chapter 5 Acids, Bases and Salts: Natural Indicators Around Us
Litmus: A natural dye

It is the most commonly used natural indicator. It is extracted from lichens. Litmus has a mauve (purple) colour in distilled water. When litmus is added to an acidic solution, it turns red. When litmus is added to a basic solution, it turns blue.
Litmus is available in the form of solution or in form of strips of paper.
Generally, it is available as red and blue litmus paper.
Activity 1
- We have mixed some distilled water with the following substances given in the table in a plastic cup/tumbler/test tube.
- Then we put a drop of the solution on a strip of the red one with the help of a dropper and repeat the same exercise with the blue litmus paper.
- We note down if there is any change in colour.
| Substance | Change in colour :- | |
| Red Litmus | Blue Litmus | |
| Lemon juice | No | Yes |
| Tap water | No | Yes |
| Detergent solution | Yes | No |
| Aerated drink | No | Yes |
| Soap solution | Yes | No |
| Shampoo | Yes | No |
| Common salt solution | No | No |
| Sugar solution | No | Yes |
| Vinegar | No | Yes |
| Baking soda solution | Yes | No |
| Milk of magnesia | Yes | No |
| Washing soda solution | Yes | No |
| Lime water | Yes | No |
We see that some solutions do not change the colour of either red or blue litmus. They are known as neutral solutions. These substances do not contain any acidic or basic substances in them.
Turmeric as an indicator
Activity 2
- We take a tablespoonful of turmeric powder. And add a little water and make a paste.
- We make turmeric paper by depositing turmeric paste on blotting paper/filter paper and drying it. Then cut thin strips of the yellow paper obtained.
- Put a drop of soap solution on the strip of turmeric paper.
We observe that:
| S. No. | Test solution | Effect on turmeric solution | Remarks |
| 1. | Lemon juice | Blue | Acidic |
| 2. | Orange juice | Blue | Acidic |
| 3. | Vinegar | Blue | Acidic |
| 4. | Milk of magnesia | Red | Basic |
| 5. | Baking soda | Red | Basic |
| 6. | Lime water | Red | Basic |
| 7. | Sugar | No change | Neutral |
| 8. | Common salt | No change | Neutral |
China Rose as Indicator
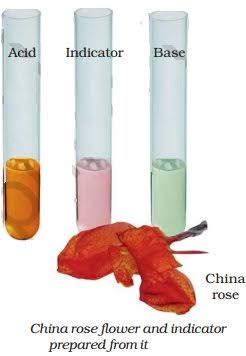
Activity 3
- We collect some China rose (Gudhal) petals and place them in a beaker.
- We add some warm water and keep the mixture for some time till water becomes coloured.
- Then we use the coloured water as an indicator and add five drops of the indicator to each of the solutions given in Table below.
- We note down the effect of the indicator on acidic, basic and neutral solutions.
China rose indicator turns acidic solutions to dark pink (magenta) and basic solutions to green.
| S. No. | Test solution | Final colour | Remarks |
| 1 | Shampoo (dilute solution) | green | basic |
| 2 | Lemon juice | pink | acidic |
| 3 | Soda water | pink | acidic |
| 4 | Sodium hydrogen carbonate solution | green | basic |
| 5 | Vinegar | pink | acidic |
| 6 | Sugar solution | neutral | neutral |
| 7 | Common salt solution | neutral | neutral |
NOTE: If you do not get the same result when using solid baking soda on dry litmus paper, make a solution of baking soda and then try.
In the table below are given some common chemicals’ tests of litmus paper, turmeric paper and china rose solution which are found in a school laboratory.
| S. No. | Name of acid | Effect on litmus paper | Effect on turmeric paper | Effect on China rose solution |
| 1 | hydrochloric acid | blue litmus red | no change | Pink |
| 2 | sulphuric acid | blue litmus red | no change | Pink |
| 3 | nitric acid | blue litmus red | no change | pink |
| 4 | acetic acid | blue litmus red | no change | pink |
| 5 | sodium hydroxide | red litmus blue | red | green |
| 6 | ammonium hydroxide | red litmus blue | red | green |
| 7 | calcium hydroxide (lime water) | red litmus blue | red | green |
Acid Rain
Rain containing excess acid is called an acid rain. The rain becomes acidic because carbon dioxide, sulphur dioxide and nitrogen dioxide (which are released into the air as pollutants) dissolve in rain drops to form carbonic acid, sulphuric acid and nitric acid respectively. Acid rain can cause damage to buildings, historical monuments, plants and animals.
NCERT Solutions for Class 7 Science Chapter 5 Acids, Bases and Salts: Neutralisation( Neutralization reaction class 7 )
We fill one fourth of a test tube with dilute hydrochloric acid and add 2-3 drops of phenolphthalein to the solution. To the acidic solution we add drops of sodium hydroxide solution by a dropper and stir it gently till the pink colour just appears.
Then we add one more drop of dilute hydrochloric acid. We observe that the solution again becomes colourless.
Again when we add one drop of sodium hydroxide solution the solution again becomes pink in colour. Since phenolphthalein gives a pink colour we come to know that the solution is basic.
But when the solution is acidic, it remains colourless.
When an acid solution and a base solution are mixed in suitable amounts, both the acidic nature of the acid and the basic nature of the base are destroyed. The resulting solution is neither acidic nor basic, i.e. it is neutral.
When we touch a test tube just after neutralisation the temperature of the test tube will be high. Because in a neutralisation reaction, heat is always produced, or evolved. The evolved heat raises the temperature of the reaction mixture.
Thus a new substance is formed. This is called salt. Salt may be acidic, basic or neutral in nature.
Neutralisation is thus defined as,
‘The reaction between an acid and a base is known as neutralisation. Salt and water are produced in this process with the evolution of heat..’
Acid+Base → Salt+Water (Heat is evolved)
For eg.
Hydrochloric acid (HCl) + Sodium hydroxide (NaOH) → Sodium chloride (NaCl) + Water (H2O)
Neutralisation in everyday life
Indigestion
Indigestion is caused when too much acid is produced in our stomach. It may be painful. To relieve indigestion, we take an antacid such as milk of magnesia, which contains magnesium hydroxide. It neutralises the effect of excessive acid and gives us relief.
Ant bite
When an ant bites, it injects the acidic liquid (formic acid) into the skin which causes pain. The effect of the acid can be neutralised by rubbing moist baking soda (sodium hydrogen carbonate) or calamine solution, which contains zinc carbonate.
Soil treatment
Plants do not grow well when the soil is either too acidic or too basic.
When the soil is too acidic, it is treated with bases like quicklime (calcium oxide) or slaked lime (calcium hydroxide).
If the soil is basic, organic matter (compost) is added to it. Organic matter releases acids which neutralises the basic nature of the soil.
Factory wastes
The wastes of many factories contain acids. When they are allowed to flow into the water bodies, the acids will kill fish and other organisms. The factory wastes are, therefore, neutralised by adding basic substances.
Exercises from textbook
1. State differences between acids and bases.
2. Ammonia is found in many household products, such as window cleaners. It turns red litmus blue. What is its nature?
3. Name the source from which litmus solution is obtained. What is the use of this solution?
4. Is the distilled water acidic/basic/neutral? How would you verify it?
5. Describe the process of neutralization with the help of an example.
More Questions
6. Mark ‘T’ if the statement is true and ‘F’ if it is false:
(i) Nitric acid turns red litmus blue. (T/F)
(ii) Sodium hydroxide turns blue litmus red. (T/F)
(iii) Sodium hydroxide and hydrochloric acid neutralize each other and form salt and water. (T/F)
(iv) Indicator is a substance that shows different colors in acidic and basic solutions. (T/F)
(v) Tooth decay is caused by the presence of a base. (T/F)
7. Dorji has a few bottles of soft drinks in his restaurant. But, unfortunately, these are not labeled. He has to serve the drinks on the demand of customers. One customer wants an acidic drink, another wants a basic one and the third one wants a neutral drink. How will Dorji decide which drink is to be served to whom?
Does nitric acid turn red litmus blue?
8. Explain why:
(a) An antacid tablet is taken when you suffer from acidity.
(b) Calamine solution is applied on the skin when an ant bites.
(c) Factory waste is neutralized before disposing it into the water bodies.
9. Three liquids are given to you. One is hydrochloric acid, another is sodium hydroxide and third is a sugar solution. How will you identify them? You have only a turmeric indicator.
10. Blue litmus paper is dipped in a solution. It remains blue. What is the nature of the solution? Explain.
11. Consider the following statements:
(a) Both acids and bases change colour of all indicators.
(b) If an indicator gives a colour change with an acid, it does not give a change with a base.
(c) If an indicator changes colour with a base, it does not change colour with an acid.
(d) Change of colour in an acid and a base depends on the type of the indicator.
Which of these statements are correct?
(i) All four (ii) a and d (iii) b, c and d (iv) only d
1. What are some different types of natural indicators?
2. What are the three categories we can divide substances into?
3. What are indicators class 7 ?
4. Sodium hydroxide turns blue litmus red true or false
5. What does calamine solution contains?
Zinc oxide: astringent
Alternative names for Calamine lotion
Ferric oxide is antipruritic